Article Text
Abstract
Background The association between serum folate deficiency and the risk of dementia in old age is unclear, perhaps owing to small sample sizes, the competing risk of mortality or reverse causation.
Objective To examine the associations between serum folate deficiency and the risks of incident dementia and all-cause mortality in a large national sample of older adults.
Methods A prospective cohort aged 60–75 years (n=27 188) without pre-existing dementia for at least 10 years, was tested for serum concentrations of folate and followed up for dementia or all-cause mortality. Serum folate deficiency was classified as present (<4.4 ng/mL), otherwise absent. HRs and 95% CIs from competing risks Cox models were fitted to quantify the associations between serum folate deficiency and the risks of dementia and all-cause mortality. To examine reverse causation, the analysis was stratified by duration of follow-up.
Findings The presence compared with the absence of serum folate deficiency was associated with higher risks of dementia (HR=1.68; 95% CI 1.32 to 2.13; p<0.001) and all-cause mortality (HR=2.98; 95% CI 2.52 to 3.52; p<0.001). Evidence for reverse causation were moderate for dementia and mild for all-cause mortality.
Conclusions Serum concentrations of folate may function as a biomarker used to identify those at risk of dementia and mortality; however, reverse causation is likely. Further research is needed to examine the role of serum folate deficiency in dementia aetiology.
Clinical implications Serum folate deficiency in older adults requires monitoring and treatment for preventative measures and/or as part of implemented therapeutic strategies.
- delirium & cognitive disorders
- adult psychiatry
Data availability statement
Data are available on reasonable request.
Statistics from Altmetric.com
Background
With ageing, serum concentrations of folate decrease,1 leading to a higher prevalence of a threshold-based medical condition termed serum folate deficiency. The highest folate deficiency rates in the population occur among older adults, with estimated prevalence rates ranging from 5% to 20%.2 3 Evidence suggests that serum folate deficiency increases the likelihood of deficits in cognitive performance and neurological functioning.4 This situates serum folate as a possible biomarker positioned to modify the risk of dementia.5 However, the few observational studies that have examined the association between serum folate deficiency and the risk of dementia have shown inconsistent results.6–11 In total, four studies have shown low serum concentrations of folate/serum folate deficiency to be associated with an increased risk of dementia,6 7 9 11 and two studies have shown a null association.8 10 With one exception,10 all studies comprised of small sample sizes,6–9 11 and no study of the association between folate deficiency and the risk of dementia has considered that mortality precludes dementia, a bias that is appropriate to account for in studies of older adults.12
Folate deficiency is associated with an elevated risk of premature mortality in the general adult population.13 Despite the increased rate of folate deficiency in older adults,1 few observational studies have scrutinised the association between serum concentrations of folate and the risk of mortality, focusing on this population segment.14 In a large population-based cohort study, serum folate deficiency was associated with an increased risk of mortality in old age,15 whereas most small-scale survey-based longitudinal studies have yielded null results.14
Prior studies of the associations between serum concentrations of folate and the risks of incident dementia or all-cause mortality have been unable to directly exclude the possibility that such associations are a by-product of reverse causation. Reverse causation has been demonstrated to be particularly relevant to studies of dementia,16 because the neuropathological processes of dementia may start decades before a formal diagnosis is given.17 Thus, plausibly, serum folate deficiency is a consequence of preclinical dementia rather than its cause.
Objective
The current study aims to examine the association between serum folate deficiency and the risks of incident dementia and all-cause mortality in a large national sample of older adults, and to scrutinize reverse causation .
Methods
Population and study design
The current study data are derived from electronic health records (EHRs) held at ‘Meuhedet Healthcare Services’ (hereafter Meuhedet), which provides healthcare services with national coverage to 14% of the total population of Israel as detailed elsewhere.18
A prospective birth cohort study design was applied (online supplemental figure 1). The eligible sample consisted of Israeli citizens from across the nation, aged 60–75 years in 2013, without pre-existing dementia (either a previous diagnosis or medication for dementia) for at least 10 years before serum folate measurements began. Individuals with pre-existing dementia were excluded from this study. All study participants were selected by their physician to be screened for serum concentrations of folate and had a least one serum folate blood test reported.
Supplemental material
Outcomes: Ascertainment of incident dementia and all-cause mortality
Outcomes were ascertained based on EHRs that include every patient with a clinical diagnosis of dementia . The diagnoses are based on the International Classification of Diseases (ICD) codes from the 9th (331.0–331.9) and 10th (F00–F03) revisions. The estimated prevalence of dementia in these EHRs is 6.6%,19 which is similar to Western Europe (6.9%) and the USA (6.5%).20 All-cause mortality was ascertained based on the registered death date. Both outcomes were assessed for a follow-up period of 58 months, from 1 January 2013 to 30 October 2017 (see online supplemental Sfigure 1 for a flow diagram).
Ascertainment of serum folate deficiency
Serum folate is a direct measure of vitamin B9 in the blood and may therefore function as a biomarker (ie, an objectively measured indicator).21 Serum concentrations of folate were measured using chemiluminescent microparticle immunoassay technology and derived from the clinical biochemistry EHRs. Measurements were ascertained as needed based on clinical impressions of the treating physician from 1 January 2013 to 30 October 2017. Serum concentrations of folate were treated as a time-dependent covariate with the follow-up time for each participant starting from their first recorded measurement. Serum folate deficiency was classified as present for concentrations below 4.4 ng/mL (<10 nmol/L), otherwise absent, based on WHO cut-off values.22
Covariates
The following study covariates were chosen to adjust for the possibility of confounding and model effect modification: background information, comorbid health conditions and folic acid supplements. The background information was birth year, sex and smoking status. Comorbid health conditions were ascertained as time-dependent covariates and classified as present from the first diagnosis onward, otherwise classified as absent. These were cognitive decline, type 2 diabetes and depression (ICD codes are summarised in online supplemental table 1). Vitamin B12 deficiency was ascertained based on serum concentrations of the vitamin from the clinical biochemistry EHRs and classified as present for serum concentrations below 150 ng/L, absent otherwise, based on previous research.23
Since serum concentrations of folate reflects recent consumption,21 exposure to folic acid supplements was included as a study covariate. Information on folic acid supplements dispensed by pharmacies nationwide was analysed based on a minimum time window that lasted 60 days and had a duration of at least 90 days. Folic acid supplement information was ascertained based on both prescription and over-the-counter purchases, using the Anatomical Therapeutic Chemical Classification System codes (ie, B03BB01 and B03BB51).
Statistical analysis
First, sample characteristics were computed to describe the differences between the presence and absence of serum folate deficiency by the study covariates. Second, rates by 10 000 person-years were estimated. Third, competing risks Cox regression modelling were used to estimate Hazard Ratios (HR) for all-cause mortality and dementia and to calculate their two-sided 95% Confidence Intervals (CI). Competing risks analysis is necessary in situations when the occurrence of one outcome (ie, mortality) precludes the occurrence of the primary study outcome (ie, dementia). In geriatric populations, the competing risk of mortality is high and can lead to invalid results.12 Accordingly, it is appropriate to account for the competing risk of mortality when examining dementia in old age. With age set as the underlying timescale, each participant was followed up to a diagnosis of dementia, death or the end of follow-up, whichever came first. The groups with and without a serum folate deficiency were compared unadjusted (including only the binary covariate of serum folate deficiency) and adjusted for the study covariates (birth year, sex, smoking status, type 2 diabetes, depression, cognitive decline, vitamin B12 deficiency and folic acid supplements). For the unadjusted model, the cumulative incidence function was estimated, and cumulative incidence curves were plotted to present the competing risks of death and dementia for the groups with and without serum folate deficiency. The analysis was weighted by the proportions of age in the national population using weights from the Central Bureau of Statistics, Israel. The proportional hazards assumption was tested with the standard statistical test. Modelling was computed in R V.3.5 using the survival library.24
Sensitivity analysis
The robustness of the results from the primary analysis was examined in three rounds of sensitivity analyses.
First, to examine reverse causation, the analysis was stratified by duration of follow-up similar to prior research.16 Based on the median follow-up cut-point, the associations between serum folate deficiency and dementia or all-cause mortality were scrutinised in the first then second half of follow-up. Serum folate deficiency was assumed to be little affected by preclinical dementia when the assessment was long before dementia onset and considerably affected when the assessment was nearer the diagnosis. Therefore, a stronger association in the first half of follow-up time may suggest that reverse causation is likely (online supplemental figure 2).
Second, the primary adjusted model was re-examined with added interaction terms to consider the effect of possible modifiers. Namely, background information (sex, smoking status), comorbid health conditions (cognitive decline, depression, type 2 diabetes and vitamin B12 deficiency) and folic acid supplements were considered. These possible modifiers are relevant to serum folate deficiency and may alone be predictive of dementia or all-cause mortality (eg, persons with cognitive decline tend to progress to clinically probable dementia at a rate five times higher than persons without it).25
Third, because the categorisation of serum folate concentrations as a threshold-based medical condition may result in a loss of information, splines were used to display the functional form of the relationship between this biomarker and dementia or all-cause mortality.
Findings
Sample characteristics
The source population consisted of 27 188 participants aged 60–75 years (M=66.26, SD=4.27). Sample characteristics displaying the differences between those with serum folate deficiency (n=3418; 12.57%) and those without serum folate deficiency (n=23 770; 87.43%) are presented in table 1. The incidence of dementia and all-cause mortality per 10 000 person-years was calculated for the aforementioned study covariates (online supplemental table 2). Among those with serum folate deficiency, the incidence of dementia was estimated at 7.96 (95% CI 3.56 to 15.32) per 10 000 person-years. Their incidence of all-cause mortality was estimated at 19.20 (95% CI 11.84 to 29.43) per 10 000 person-years. Among those without serum folate deficiency, the incidence of dementia was estimated at 4.24 (95% CI 3.34 to 5.30) per 10 000 person-years. Their incidence of all-cause mortality was estimated at 5.36 (95% CI 4.34 to 6.54) per 10 000 person-years.
Sample characteristics
Serum folate deficiency and the competing risks of dementia and all-cause mortality
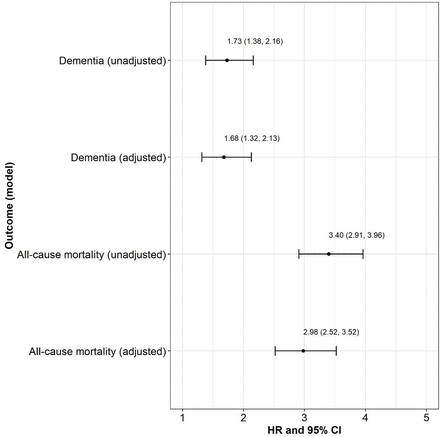
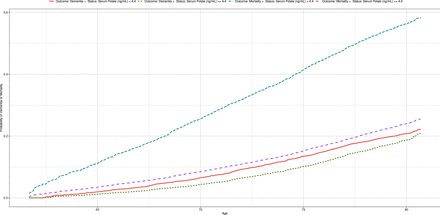
There was no deviation from the proportional hazards assumption for the association between serum folate deficiency and the risks of dementia (χ2=0.82, p=0.37) and all-cause mortality (χ2=0.02, p=0.90) for any study covariate (online supplemental table 3). The presence compared with the absence of serum folate deficiency was associated with a higher risk of dementia (unadjusted: HR=1.73; 95% CI 1.38 to 2.16; p<0.001; adjusted: HR=1.68; 95% CI 1.32 to 2.13; p<0.001) and all-cause mortality (unadjusted: HR=3.4; 95% CI 2.91 to 3.96; p<0.001; adjusted: HR=2.98; 95% CI 2.52 to 3.52; p<0.001; figure 1, online supplemental table 4). Figure 2 presents the cumulative incidence curves of dementia and all-cause mortality, indicating that the presence of serum folate deficiency was associated with higher rates of dementia and all-cause mortality compared with the absence of serum folate deficiency.
The association between serum folate deficiency and the risks of dementia and all-cause mortality. Note: HR: HR from the Cox regression model; CI: Wald two-sided 95% CI; p value: p value for test of the hypothesis HR=1 versus the hypothesis HR≠1. Reference group=serum folate deficiency absent; non-reference group=serum folate deficiency present.
Cumulative incidence curves of dementia and all-cause mortality
Sensitivity analyses
The robustness of the associations between serum folate deficiency and the competing risks of dementia and all-cause mortality was re-evaluated in three rounds of sensitivity analyses.
First, in sensitivity analyses stratified by follow-up time, the association between serum folate deficiency and the risk of dementia was significant in the first half of follow-up (HR=1.74; 95% CI 1.36 to 2.21; p<0.001) and null in the second half of follow-up (HR=0.98; 95% CI 0.46 to 2.09; p=0.97) (online supplemental table 5). For mortality, the association was significant throughout the follow-up time, although stronger in the first than second half of follow-up (online supplemental table 5). These results provide evidence for reverse causation in the association between serum folate deficiency and dementia or all-cause mortality.
Second, the primary model with added interaction effects generally did not attenuate the association between serum folate deficiency and the risk of dementia (online supplemental table 6). Moderation effects were observed in some interactions tested with all-cause mortality (online supplemental table 6).
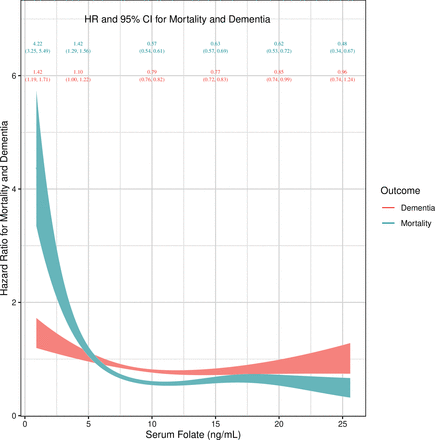
Third, the association between serum folate deficiency and the competing risks of dementia and all-cause mortality was not attenuated in sensitivity analysis calculated using splines (ie, serum concentrations of folate measured continuously). The HRs for dementia or all-cause mortality declined as serum folate concentrations increased (figure 3).
Serum folate concentrations and dementia or all-cause mortality
Discussion
The current study examined the association between serum folate deficiency and the risks of dementia and all-cause mortality among older adults in the largest prospective cohort to date. The results suggest that serum folate is a biomarker that may be used to modify the risks of dementia and mortality in old age. Serum folate deficiency (<4.4 ng/mL) was associated with a 1.68-fold increased risk of dementia and a 2.98-fold increased risk of all-cause mortality. Generally, across sensitivity analyses of interactions, the results were not significantly attenuated. However, the role of serum folate deficiency in dementia aetiology was questioned due to evidence of reverse causation.
The current study result that serum folate deficiency is a medical condition associated with a higher risk of incident dementia is in line with some prior studies of Alzheimer’s disease6 and dementia.9 Tentative mechanisms explaining this association are that folate deficiency affects homocysteine level and therefore the vascular risk of dementia10 and/or that folate deficiency affects impaired DNA repair in neurons, sensitising them to oxidative damage,26 which may accelerate age-related dysfunction and cell damage.27 However, the current study provides moderate evidence for reverse causation, suggesting that serum folate deficiency may be a consequence of preclinical dementia rather than its cause.
The current study results are consistent with some, but not other, evidence demonstrating that serum folate deficiency is associated with an increased risk of mortality.15 Folate deficiency may affect the risk of all-cause mortality by failing to prevent heart disease and cancer, through tentative mechanisms of DNA damage and inhibited repair and/or increased malignant transformations,26 which increase with age.27 However, evidence provided by the current study suggest that reverse causation likely plays a role in the association between serum folate deficiency and all-cause mortality. Further work (eg, midlife longitudinal studies) is required to establish whether the observed associations are causal.
Limitations
Our study has notable limitations. A major limitation of this study relates to the selection of the population. While this study was based on a very large national cohort, it only focused on individuals who were selected to be screened for serum concentrations of folate. Measurements were ascertained as needed based on clinical impressions of the treating physician, which may have induced bias. However, in the current data, the prevalence of serum folate deficiency was estimated at 12.57%, which is similar to prior population-based research.3 Nevertheless, future studies ought to examine an unselected representative sample. In terms of generalisability, we focused on individuals aged 60–75 years due to data availability limitations. This restricts our ability to extrapolate from our results to the broad elderly population.
Another limitation relates to the observational study design that has limited implications for causality. Indeed, stratified follow-up analyses suggest that reverse causation may play a role in the association between serum folate deficiency and the risks of dementia or all-cause mortality. Hence, the issue of reverse causation requires further examination via midlife longitudinal studies.
Additionally, serum concentrations of folate may not be indicative of long-term folate status because of sensitivity to fluctuations in recent food intake and metabolism.21 Nonetheless, serum folate deficiency remains an indicatory measure, as it is considered the first evidence of a negative folate balance.28 Ascertainment of dementia may also be subject to bias, and it is probable that incident dementia was underascertained. On the other hand, folate deficiency may lead to reversible dementia which may be incorrectly classified as irreversible dementia. 29 While reversible dementia due to folate deficiency is relatively uncommon, existing research on this topic is based mainly on case reports and a single retrospective study29 and thus warrants further investigation. Still, the overall rate of dementia in our data is similar to other nations.20
Finally, it may be that serum folate deficiency is associated with dementia and mortality owing to certain genetic risk factors undetected in the current study (eg, APO-E e-4 allele).6 However, the covariates chosen for the current study were based on the main conditions previously found to be associated with folate deficiency, dementia and all-cause mortality (eg, type 2 diabetes). Still, genetic risk factors may be the next step forward in future research.
Despite these limitations, our study used a large-scale nationwide prospective cohort of older adults without pre-existing dementia for at least 10 years. We examined multiple serum folate measurements and the competing risks of incident dementia and all-cause mortality while controlling for established risk factors. Generally, the results point to consistent associations between serum folate deficiency and higher risks of dementia and all-cause mortality, suggesting that serum folate is a biomarker that may play a pivotal role in ageing. However, ours is the first study to query whether reverse causation could explain these associations. Indeed, evidence for reverse causation were moderate for dementia and mild for all-cause mortality, suggesting that further research is needed to examine the role of serum folate deficiency in dementia aetiology.
Clinical implications
Serum concentrations of folate may function as a biomarker used to modify the risks of dementia and mortality in old age. The implications for public health policy appear to be to reliably monitor serum concentrations of folate in older adults and treat deficiency for preventative measures and/or as part of implemented therapeutic strategies while regularly reviewing patients’ clinical outcomes.30
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
The Institutional Review Board at the University of Haifa and the Meuhedet associated Helsinki Institutional Review Board granted ethical approval to conduct the current study with a waiver of written informed consent.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @Arotstein, @szlevine
Contributors AR, AK, ARE and SZL designed the study; AR, YG and SZL analysed the data; AR drafted the manuscript; and AR and SZL wrote the manuscript. All authors provided critical manuscript feedback.
Funding AR is supported by the Zuckerman-CHE Israeli Women Postdoctoral Scholarship.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.